Influenza, also known as the flu, is a highly contagious respiratory illness that affects millions worldwide yearly. With the potential to cause severe illness and even death, early detection and management of influenza is crucial. This is where Influenza A/B Test Kits come in. These simple yet powerful tools are designed to quickly and accurately detect the presence of Influenza A or B viruses in the body, allowing for prompt treatment and prevention of further spread. In this blog post, we will delve into the details of Influenza A/B Testing Kits and how they can aid in the fight against this infectious disease.
Understanding Influenza A and B
Influenza A and B are the principal strains responsible for seasonal flu epidemics globally. Influenza A, with its ability to infect humans and animals, poses a significant threat due to its potential to cause widespread outbreaks. This strain is notorious for its capacity to change, leading to new subtypes that can trigger pandemics. On the other hand, Influenza B’s impact is mainly confined to the human population.
It tends to result in milder epidemics but can cause considerable illness and disruption. Both strains manifest through a constellation of symptoms that can severely impact individuals’ health, productivity, and quality of life. These include high fever, aches, sore throat, and extreme fatigue, which can hinder affected individuals.
Given their capacity to mutate and the similarities in their clinical presentations, distinguishing between these two types without specific testing is challenging. This underscores the importance of accurate diagnostic tools, such as the flu A/B Testing Kits, in effectively managing and controlling the spread of the virus.
How Do Influenza A/B Testing Kits Work?
Influenza A/B Testing Kits utilise a sophisticated yet user-friendly technology to detect the presence of influenza viruses within individuals’ respiratory samples. The core technique employed by these kits is the rapid antigen detection test (RADT), which specifically targets antigens from the influenza viruses.
Upon collection of a nasal or throat swab from a person exhibiting symptoms of influenza, the specimen is treated with a reagent provided within the kit. This reagent reacts with the sample so that if influenza antigens are present, a visible indication will appear on the test device, often within minutes. This method does not require sophisticated equipment or extensive time associated with polymerase chain reaction (PCR) tests, offering a practical and sensible means for healthcare practitioners to identify the influenza virus.
It’s pivotal to acknowledge, however, that while this process facilitates rapid diagnosis at the point of care, it should always be performed using the specific instructions included with the test kit to ensure the accuracy and reliability of the results.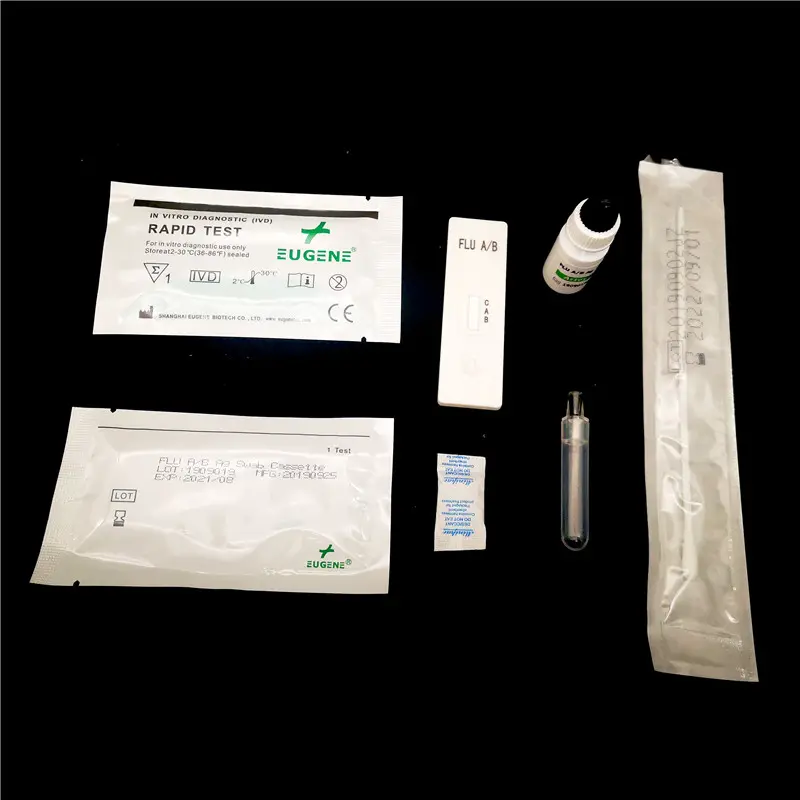
Accuracy and Reliability of Testing Kits
Flu A/B Testing Kits are renowned for their precision in identifying influenza viruses, exhibiting a commendable balance of sensitivity and specificity. Comparative studies have illuminated that the accuracy of these kits closely aligns with that of more sophisticated laboratory-based PCR tests. This high level of reliability is contingent upon strict adherence to the manufacturer’s guidelines.
Missteps in the execution of the test, including improper collection of the specimen or deviation from prescribed procedures, can compromise the integrity of the results. Therefore, it is imperative that users meticulously follow the instructions provided with the kit to safeguard the accuracy of the findings.
Moreover, while these kits are adept at confirming the presence of influenza viruses, healthcare practitioners may occasionally recommend confirmatory testing through laboratory methods, especially in cases where clinical symptoms persist despite a negative test result. This layered approach to diagnosis ensures that individuals receive the most accurate assessment of their health status concerning influenza infection.
The Role of Flu A/B Testing Kits
Flu A/B Testing Kits serve a multifaceted role in managing and controlling influenza outbreaks. Their rapid, accurate diagnosis capabilities enable healthcare professionals and individuals to make informed decisions regarding treatment and prevention. The following points elaborate on the distinct roles these kits play.
Early Diagnosis
By facilitating the early detection of influenza types A and B, these kits allow for the swift initiation of appropriate medical interventions, potentially reducing the severity and duration of the illness.
Reduction in Transmission
Early identification of the flu virus aids in implementing measures to prevent its spread, such as isolation and enhanced hygiene practices.
Resource Allocation
In healthcare settings, using these kits can help prioritise the allocation of resources, including antiviral medications and hospital beds, especially during peak flu seasons.
Public Health Monitoring
Flu A/B Testing Kits contribute to the surveillance of influenza trends, helping public health officials monitor and respond to outbreaks with targeted interventions.
Personal and Community Peace of Mind
Knowing one’s flu status can alleviate personal anxiety and reduce the spread of the virus, contributing to the overall health security of communities.
The Cost Factor
The financial aspect of flu A/B Testing Kits is a critical consideration, particularly when evaluating the broader accessibility and implementation of these diagnostic tools. Priced more affordably than many laboratory-based diagnostics, these kits present a cost-effective solution for healthcare systems and individuals.
The lower cost does not compromise the quality or accuracy of the results, offering a viable alternative for prompt influenza detection. This economic efficiency extends the reach of influenza testing, ensuring more people can access timely diagnosis without incurring significant expense.
Available widely in chemists and clinics, the Testing Kits enhance the feasibility of conducting tests outside traditional healthcare settings, thereby reducing the burden on healthcare facilities and streamlining the management of influenza cases. The affordability of these kits is instrumental in fostering a more proactive approach to influenza testing, which is essential for effective outbreak management and prevention strategies.
Reading and Interpreting Results
Interpreting the results from flu A/B Testing Kits is designed to be a clear and straightforward process, ensuring individuals can easily understand their health status regarding influenza infection. Upon conducting the test, a positive result signifies that the influenza virus, either A or B, has been detected in the specimen. This indication necessitates prompt communication with a healthcare professional to discuss the next steps in treatment and care.
Conversely, a negative result suggests the influenza virus is absent in the collected sample. However, individuals must be aware that a negative result does not entirely rule out the possibility of an influenza infection. Factors such as the timing of the test, the onset of symptoms, and the specific characteristics of the virus strain may affect the outcome.
Therefore, in instances where symptoms persist or worsen, it is advised to seek further medical consultation. This approach ensures that the reading and interpretation of test results are used effectively as part of a broader diagnostic and treatment strategy.
What to Do After Testing Positive
Upon receiving a positive result from a flu A/B test kit, it is paramount for the individual to consult with a healthcare provider immediately. This consultation will typically lead to a recommendation for antiviral medicines designed to lessen the severity of influenza symptoms. Rest and an adequate intake of fluids are also advised to support recovery.
Adopting stringent respiratory hygiene practices is essential to curb the transmission of the virus. This includes using tissues or the crook of the elbow to cover the mouth and nose when coughing or sneezing and disposing of tissues in a sealed bin immediately after use. Frequent handwashing with soap and water or using alcohol-based hand sanitisers reduces the risk of spreading the virus.
Isolating one from others, particularly in shared living and working spaces, minimises the potential for influenza to infect family members, colleagues, and the broader community.
The Benefits of Early Detection
The advantages of early detection of influenza through the use of flu A/B Testing Kits are manifold. Identifying the virus at the onset of symptoms can lead to more effective illness management, lessening its severity and duration. Below are the key benefits outlined:
Prompt Treatment Initiation
Early diagnosis allows for the immediate commencement of antiviral medications, most effective when started within the first 48 hours of symptom onset.
Improved Patient Outcomes
Detecting the flu early can significantly reduce the risk of severe complications, such as pneumonia, particularly in high-risk groups.
Decreased Spread of the Virus
Knowing one’s flu status early helps take timely measures to avoid spreading the virus to others, protecting family, friends, and the broader community.
Reduced Healthcare Burden
Early detection and treatment can lessen the demand for healthcare services by reducing the number of severe cases and hospitalisations.
Enhanced Surveillance and Response
Early detection contributes to the tracking of influenza outbreaks on a larger scale, allowing for more effective public health responses and resource allocation.
Limitations of Flu A/B Test Kits
Despite their invaluable role in rapidly detecting influenza viruses, it is imperative to acknowledge certain limitations associated with Flu A/B Test Kits. One notable constraint is the variable sensitivity across different strains of influenza, which can sometimes lead to false-negative results, especially when new strains emerge or peak flu seasons occur.
The limitation underscores the importance of considering clinical symptoms and the potential need for additional testing in cases where influenza infection is strongly suspected despite a negative test outcome. Additionally, these kits are designed for early detection; hence, their effectiveness may diminish if the test is administered too late during the infection. Moreover, environmental factors and improper handling or storage of the Testing Kits can adversely affect the accuracy of the results.
It is also critical to understand that flu A/B Testing Kits do not provide information on the virulence or resistance patterns of detected strains, which could be vital for guiding treatment decisions. Recognising these limitations is crucial for healthcare practitioners and individuals to utilise these kits effectively within the broader context of influenza diagnosis and management.
Preventative Measures beyond Testing
Beyond the utilisation of flu A/B Testing Kits, several additional precautions are recommended to mitigate the risk of contracting and spreading influenza. Annual vaccination is the foremost preventative measure, protecting against the most prevalent flu strains expected each season.
Adherence to robust hand hygiene practices, including regular washing with soap and water or applying alcohol-based hand sanitisers, is crucial in limiting transmission. Individuals are advised to maintain a safe distance from those exhibiting illness symptoms and exercise caution in shared spaces.
Furthermore, the importance of self-isolation when experiencing flu-like symptoms cannot be overstated, as it prevents the potential spread to others. Adopting these preventative strategies collectively enhances the resilience of communities against the influenza virus, complementing the diagnostic benefits provided by flu A/B Testing Kits.
Conclusion
In summary, Influenza A/B Test Kits represent a pivotal advancement in the early detection and management of influenza. Through quick and accurate results, these kits facilitate timely medical interventions and help curtail the spread of the virus. Despite certain limitations, when employed correctly and as part of a comprehensive approach to health, they stand as a formidable tool in the fight against influenza. However, individuals must also adopt preventive measures such as vaccination and good hygiene practices to bolster their defence against this ever-present threat.
FAQs
What is the primary function of flu A/B Testing Kits?
These kits are designed to rapidly detect influenza types A and B in individuals, enabling timely diagnosis and management of the illness.
How are Flu A/B Test Kits operated?
Flu A/B Test Kits utilise rapid antigen detection technology, analysing respiratory samples through a simple process to ascertain the presence of influenza viruses.
How reliable are flu A/B Testing Kits in identifying influenza viruses?
With a notable degree of sensitivity and specificity, these kits offer a high level of accuracy, closely paralleling that of laboratory PCR tests, provided the manufacturer’s instructions are strictly followed.
What actions should be taken following a positive test result from a flu A/B test kit?
Immediate consultation with a healthcare provider is advised to commence suitable treatment and discuss preventive measures to avoid spreading the virus.
Can flu A/B Testing Kits be an alternative to the flu vaccine?
No, these Testing Kits are diagnostic tools, not replacements for the protective benefits afforded by annual flu vaccinations.
| Other Good Articles to Read |
| Cme Blog Spot |
| Garcias Blogs |
| Yyc Blogs |
| Guiade Blogs |
| Blogs-Hunt |
| Impact-Blog |
| Smarty Blogs |
| Ed Blog |
| Mo Blogs |
| Blogs Em |
| Blog St |
| Related Business Listings |
| Contact Directory |
| Local Business Profiles |